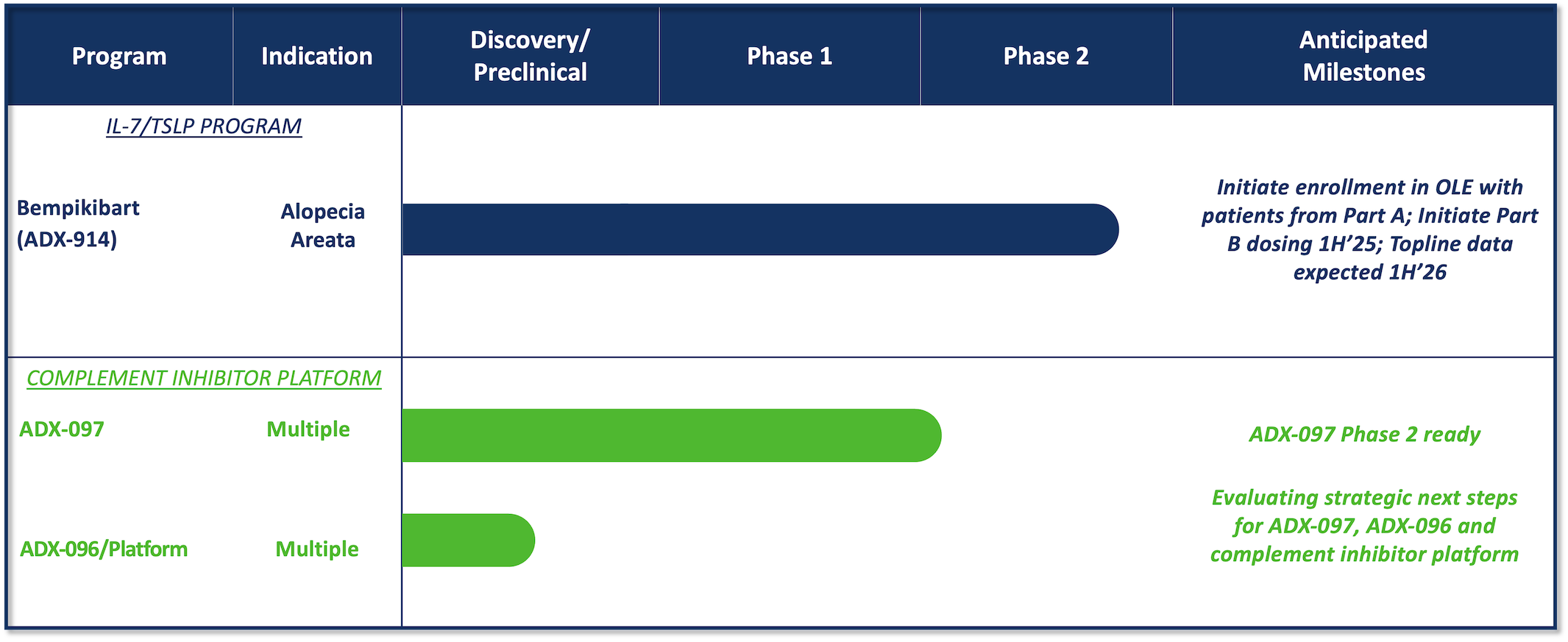
Our Pipeline
Our clinical and discovery pipeline programs are centered on restoring immune homeostasis in patients with severe inflammatory and autoimmune diseases.

For pipeline inquiries, please contact: BD@q32bio.com.

IL-7/TSLP Program
Bempikibart (ADX-914) showed promising clinical activity in Q32 Bio’s Phase 2a clinical trial. Bempikibart was observed to have a well-tolerated safety profile, pharmacokinetic/pharmacodynamic results at desired exposures, inhibition of Th2 and Th1 biomarkers consistent with expected target engagement, and encouraging clinical activity in patients with alopecia areata (AA). Q32 Bio is currently evaluating bempikibart in patients with AA in the ongoing SIGNAL-AA Phase 2a clinical trial.
Bempikibart is a fully human anti-IL-7Rα antibody that is designed to re-regulate adaptive immune function by blocking IL-7 and TSLP signaling via their cognate receptors. IL-7 and TSLP signaling have been biologically linked to numerous inflammatory and autoimmune diseases.
In AA, Th1 has long been implicated in the pathogenesis of AA supporting the potential for bempikibart to directly address the underlying driver of follicle damage and hair loss. In addition, given that AA is a disease often diagnosed in young adults, there is a critical need for effective novel treatments with a safety profile suitable for long-term, chronic treatment.

Complement Program
Q32 Bio is currently evaluating strategic options for our Phase 2, tissue-targeted complement inhibitor, ADX-097, and our complement inhibitor platform which includes the development candidate ADX-096, a C3d – CR1 fusion protein, and other earlier stage assets, including C3d mAb fusions and nanobodies designed for tissue-targeted complement inhibition. Q32 Bio has developed a novel discovery platform that is enabling the advancement of tissue-targeted complement inhibitors which have potential therapeutic activity across the numerous diseases in which C3 complement fragments are deposited in diseased tissue. ADX-097 has the potential for the treatment of renal and other complement-mediated diseases of high unmet need, including lupus nephritis (LN), IgA nephropathy (IgAN), and C3 glomerulopathy (C3G).
In preclinical studies, ADX-097 distributed to affected tissues/organs and demonstrated durable tissue pharmacokinetics and pharmacodynamics. Additionally, Q32 Bio has evaluated ADX-097 in a Phase 1 clinical trial in healthy volunteers where Q32 Bio observed circulating PK/PD consistent with preclinical studies, which established in vivo ADX-097 integrity and informed Q32 Bio’s dosing strategy for next stage clinical testing. ADX-097 was also shown to be well tolerated.
Based on the compelling evidence from these studies, Q32 believes ADX-097 has the potential to address the limitations of the currently available systemic approaches to complement inhibition, including infection risk and the need for high drug doses and frequent administration, to achieve therapeutic levels of inhibition.